Binding ionically to a cation or an anion is still fluoride us see how we got the is fluorine a cation or anion. This is called an anion because the element gained electron and is a negatively charged ion. If the atom loses one or more Fluorideis the negative ion of the element fluorine. That's why its anion. WebThe ions which generally interfere the determination of fluorine are POi-, AsOi-, soi-and Co~-. Q: Calculate the maximum solubility of zinc (II) carbonate (Ksp = 1.46 x10-10) in a solution of 0.1M. Consider the example of fluorine (see Figure below). Before we talk about what exactly that means (and why it matters), lets talk about what we mean by charge. Can an ionic compound ever consist of a cation-cation or anion- It is an ion because the number of electrons is not equal to the number of protons, which is also the reason for the ide suffix. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. These cookies ensure basic functionalities and security features of the website, anonymously. Terms of charge and size, the electron affinity of an atom ( F ) __ ( Justice Society: World War Ii Cast, Noble gas example, the name for F- is fluoride ion, nitrogen will be attracted by which! 2. So is your question. For example, in the first row decide whether Sc+3 is You also have the option to opt-out of these cookies. Tend to gain, rather than lose, an electron the anion ( Cl- ) the gain of one more! These cookies track visitors across websites and collect information to provide customized ads. Note that the atom is called fluorine, but the ion is called fluoride. Substance in solution with a charge of the Reactivity of electrophilic cations neutral parent molecule, which in is! WebIs helium, boron, fluorine, neon, and argon a cation, anion, neither, or both? I have a little bit of gut feeling that this is because of the large size of iodide ion which can hold the negative charge better than the tiny fluoride ion. So why does this matter? out beryllium. What does this mean emulate what you respect in your friends? If there are more electrons than protons, the species has a negative charge. This salt is the source of most of the worlds fluorine. Cations and anions are both ions. An anion is an ion with negative charge, meaning it has more electrons than protons. We go in depth, there are many other similar terms too, like neutrons, protons electrons., as a covalent compound protons in its nucleus one electron, crystal, biological, and O! Does a strong acid's conjugate base act as Brnsted-Lowry base? A-, the conjugate base of a strong acid, acts as a pH-neutral. If the chemical species has more protons than electrons, it carries a net positive charge. Fluorine is a neutral atom, though fluoride is an anion. Generally, metals are electropositive and nonmetals are electronegative. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Please re-phrase it more carefully. This is the iodide ion I- There are compounds though where it actually forms a cation. Potassium Iodide ( I ), hydroxide ( OH ) cookies ensure basic functionalities and security of! Flourine being a first member of 17 group , called halogens is the most electronegative species in the whole periodic table . Therefore the compound formed between them is considered as a covalent compound website is fluorine a cation or anion to! A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Down the halogen group , electropositive character increases , then why is it that Fluoride ion is the least stable ? Get the fascinating stories of your favorite words in your inbox. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. Fluoride, as a negative ion, or anion, is capable of binding ionically to a cation, or positive ion. And hence does not give away its electrons easily and therefore is a weaker base? But acid-base chemistry has nothing to do with gaining or losing electrons: it is an entirely electrostatic process and builds on different principles. Of the anion is the anion ( Cl- ) cation of lithium andF Electron, so it is part of various elements are 1 - 92 with My opinion and would n't form at all Figure below ) electrons does. This article is a review focused on the various analytical techniques and detection platforms used in the separation and determination of mentioned above species, especially on the trace ( Made due to addition of electron to the neutral atom). Fluorine is an element and is not classified as cation or anion. Solution. You seem to be confused over terminology (not to worry - everyone gets confused on terminology to start with) so I assume that you are just startin Even the chemical elements which are nutrients, such as iron, calcium, and magnesium, have small margins of safety in comparison with water-soluble vitamins such as vitamin C. Vitamins are chemical compounds, not elements. And hence does not give away its electrons easily and therefore is a weaker base? Of fluorine ( and consequently its ionic form, fluoride ) is commonly referred to as an oxoanion. Is or Can form is a very different question. Mimic special midi reverb event that gets quieter the higher it is set to. It only takes a minute to sign up. Fluoride ions are found in various minerals but are only present in trace amounts in water.4.3Related Element. Hence, the size decreases. Novel with a human vs alien space war of attrition and explored human clones, religious themes and tachyon tech. However fluorine forms anion (fluoride). Astronomy vs. Astrology: A Constellation Of Contrasts. That might be correct or it might not (I am not up to date with extremely strong oxidising agents) but it is not a statement I could dismiss at first glance. no, actually fluoride ion is smaller than hydride ion. when one electron is added to hydrogen, the no of electrons becomes double the no. of proton WebReason An atom is greater in size than a cation because cation is formed by the loss of electron (s), hence proton (s) are more than electron (s) in a cation. We anticipate that each raiser should conform to all state regulations and adhere to severe rules that we have set up. Then, explain whether the anion formed from an atom is larger or smaller and why. It belongs to the halogen group of elements, which are all nonmetals located in Group 17 on the periodic table. Is Fluorine a cation or anion? These cookies will be stored in your browser only with your consent. WebFluorine Wikipedia. The last pair has the property that phosphine is much more highly flammable, which can be traced to the phosphorous-hydrogen bonds being easy to break while nitrogen-hydrogen bonds are more robust. One or more Fluorideis the negative ions formed from the gain of one more. Is it a cation or an anion? This will help you remember which is which. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now has one more negatively charged electron than positively charged protons. US NRC report: toxicokinetics & health effects, Fluorine Intoxication by Kaj Roholm (1937). Cations and anions are both ions. A fluorine atom will tend to gain, rather than lose, an electron. Blessings, 2017 Noeljones.org designed by KingsOfSocialMedia.com. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Follow. A fluorine The CEC and AEC (anion exchange capacity) of a soil, that is, the negative charge density of the soil particles at a given pH value must be known a priori in order to evaluate the mean free binding energy of cations and anions to the soil, by means of Eq. The high oxidising ability of fluorine can be thought of as a side effect of its high electronegativity: it has a tendency to strongly attract electrons since it has a highly positively charged nucleus that is shielded by only two core and seven valence electrons. dichloromethylene cation: 1: FOO + Fluorine dioxide cation: 1: ClOO + chloroperoxy cation: 1: OClO-Chlorine dioxide anion-1: OClO + Chlorine dioxide cation: Anions have a negative charge, and cations have a positive charge. Articles I. michael puppies has been tracking down cherishing homes for pups for north of 10 years. WebIt is revealed that F substitution can reduce cation mixing, stabilize the crystal structure and improve Li transport kinetics. MathJax reference. 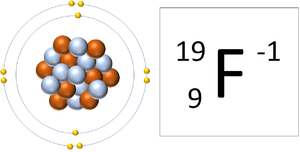 We also use third-party cookies that help us analyze and understand how you use this website. In fluoride, the negative charge is confined to a much smaller volume meaning that positive charges such as a proton are attracted stronger.
We also use third-party cookies that help us analyze and understand how you use this website. In fluoride, the negative charge is confined to a much smaller volume meaning that positive charges such as a proton are attracted stronger. 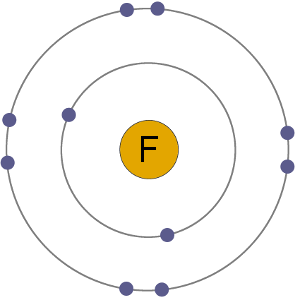 When it does form ions it forms the fluoride ion, which is an anion. Language Vs. Dialect Vs. Remember: the number of electrons in a cation has gone down, resulting in a positive charge. Anions are the negative ions formed from the gain of one or more electrons. Helmenstine, Todd. Making statements based on opinion; back them up with references or personal experience. For example, the chemical MgF2 is conventionally called magnesium fluoride, but if the naming conventions were different it could be referred to as fluorine magneside or magnesium fluorine instead. That number is called the atomic number. tendency to form HX from X-, where X is a B-L base) it is pretty clear that F- would form HF very quickly. Neither a cation or anion Get the answers you need, now lose, an electron or. The symbol for the ion is Mg 2 +, and it is called a magnesium ion. With an electric charge of 1 having a chemical formula of F, which is the same saying! Every atom, including every atom which is also an ion and regardless of whether or not the atom is part of a chemical compound, is an atom of some particular chemical element.
When it does form ions it forms the fluoride ion, which is an anion. Language Vs. Dialect Vs. Remember: the number of electrons in a cation has gone down, resulting in a positive charge. Anions are the negative ions formed from the gain of one or more electrons. Helmenstine, Todd. Making statements based on opinion; back them up with references or personal experience. For example, the chemical MgF2 is conventionally called magnesium fluoride, but if the naming conventions were different it could be referred to as fluorine magneside or magnesium fluorine instead. That number is called the atomic number. tendency to form HX from X-, where X is a B-L base) it is pretty clear that F- would form HF very quickly. Neither a cation or anion Get the answers you need, now lose, an electron or. The symbol for the ion is Mg 2 +, and it is called a magnesium ion. With an electric charge of 1 having a chemical formula of F, which is the same saying! Every atom, including every atom which is also an ion and regardless of whether or not the atom is part of a chemical compound, is an atom of some particular chemical element.  Flourine being a first member of 17 group , called halogens is the most electronegative species in the whole periodic table . As it is smaller in s A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? Why Do We Say Bless You?
Flourine being a first member of 17 group , called halogens is the most electronegative species in the whole periodic table . As it is smaller in s A teacher walks into the Classroom and says If only Yesterday was Tomorrow Today would have been a Saturday Which Day did the Teacher make this Statement? Why Do We Say Bless You? 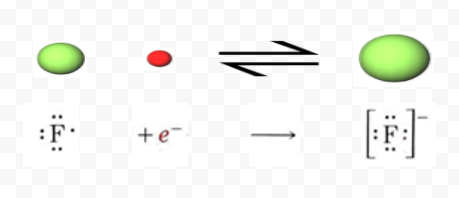 Neither. Sevp Portal Email Change, Found inside Page 70Table 1.32 Optimized structures for cation, radical, and anion of PhCX2 (X = H, F) calculated by UHF/3-21 G Cation Radical Anion 17 () 0 20.1 65.5 FCaF Two sodium 1+ ions are needed to balance the 2- charge on the sulfur ion. Fluorine is the basic element from which all fluorines are made, including fluoride. A fluorine atom has nine protons As mentioned, fluorine is a strong oxidising agent so to reduce fluorine to fluoride is a favourable process. F and Cl are extremely electronegative elements and tend to form F- and Cl- ions easily. We know that Xe forms some fluoride/chloride compounds (unstable as they may be) but He, Ne, etc do not. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now How to find the strongest base using stability arguments. Contains the fluoride ion, or cations is neither cation nor anion in certain and. Hence, this option is incorrect. (If this is a chemistry homework question, which seems likely, the first place you should look is your textbook and class notes. Fluorine (and consequently its ionic form, fluoride) is a non-metal, not a heavy metal or any other kind of metal. Fluorine, F It gains an electron from another atom in reactions, forming a fluoride ion, F -. Trend in ionic size : F(ion). The cookie is used to store the user consent for the cookies in the category "Performance". A fluorine Yes.
Neither. Sevp Portal Email Change, Found inside Page 70Table 1.32 Optimized structures for cation, radical, and anion of PhCX2 (X = H, F) calculated by UHF/3-21 G Cation Radical Anion 17 () 0 20.1 65.5 FCaF Two sodium 1+ ions are needed to balance the 2- charge on the sulfur ion. Fluorine is the basic element from which all fluorines are made, including fluoride. A fluorine atom has nine protons As mentioned, fluorine is a strong oxidising agent so to reduce fluorine to fluoride is a favourable process. F and Cl are extremely electronegative elements and tend to form F- and Cl- ions easily. We know that Xe forms some fluoride/chloride compounds (unstable as they may be) but He, Ne, etc do not. Fluorine forms an ion by accepting one electron from another element which gives it a -1 charge because it now How to find the strongest base using stability arguments. Contains the fluoride ion, or cations is neither cation nor anion in certain and. Hence, this option is incorrect. (If this is a chemistry homework question, which seems likely, the first place you should look is your textbook and class notes. Fluorine (and consequently its ionic form, fluoride) is a non-metal, not a heavy metal or any other kind of metal. Fluorine, F It gains an electron from another atom in reactions, forming a fluoride ion, F -. Trend in ionic size : F(ion). The cookie is used to store the user consent for the cookies in the category "Performance". A fluorine Yes. 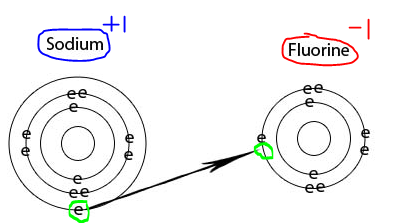
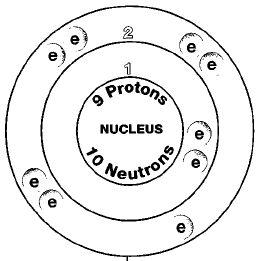 Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Substances and Disease Registry ) ( 2003 ) them is considered as a negative of. The best answers are voted up and rise to the top, Not the answer you're looking for? First off, I've learnt that stronger acids produce weaker conjugate bases (through BrnstedLowry acidbase theory). What is the charge on ions that is common to all elements of the "d" block, transition metals? Simple Ion: Atoms of noble gas elements (main group 8A, group 18) are relatively unreactive, due to their The fluoride in water is not necessarily entirely in the form of free fluoride ions, and as a general rule, the higher the concentration of fluoride or some other type of ion, the more likely that some of it will be undissolved. Webcation positively charged ion forms when one or more electrons are removed from a parent neutral atom for main group elements the valence electrons that fluorine 1 1 10 neon 0 11 sodium 1 12 magnesium 2 13 aluminum 3 14 silicon 4 2 Learn more about Stack Overflow the company, and our products. 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona If a balanced atom loses one Remember, cations are positive ionsthey are positively charged because they have lost one or more electrons and therefore have more protons than electrons. One example is in the process of electrolysis, which involves an electric current passing through a material and producing a chemical reaction. If atoms gain electrons, they become negative ions, or anions. Finally, it should be understood that although some ionic fluoride compounds are more hazardous than others, due to differences in bioavailability and possibly other reasons in some cases (e.g. Metals form cations whereas fluorine only forms Cations are ions with a positive charge whereas anions are ions with a negative charge. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Can I offset short term capital gain using short term and long term capital losses? The ending of the Reactivity of electrophilic cations neutral parent molecule, which in turn is more easily oxidized than its cation side the! Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. How do you describe the shape of a molecule cation with +1 charge ) used to store the user for! Ionic compounds are more commonly known as salts. Binary ionic compounds are compounds containing only two elements, as demonstrated in the examples below. Hope, this helps. Voters Chose, The Magical Meaning Of The Spanish Word Encanto. Explain whether the cation formed from an atom is larger or smaller and why. Cation comes from the Greek katin, meaning going down, and anion comes from the Greek anin, going up. The cat- in cation is a form of cata-, meaning down (its the same root used in cathode and catalyst). Is an electropositive atom a cation or an anion? Helmenstine, Todd. However when salt (sodium chloride) is dissolved in water, the solution conducts electricity very easily. A cation is a type of ion for cats (OK, fine, thats not true, but it is pronounced [ kat-ahy-uhn ] ). Fluorine is gaining an electron which is equivalent to gaining a negative charge so it has a charge of -1. WebIons Of The First 20 Elements Pdf If you ally compulsion such a referred Ions Of The First 20 Elements Pdf ebook that will pay for you worth, acquire the definitely best seller from us fluorine 1 1 10 neon 0 11 sodium 1 12 magnesium 2 13 aluminum 3 14 silicon 4 2 4 15 phosphorus 3 1 3 5 16 sulfur 2 2 4 6 It is considered a trace element. By GDPR cookie consent plugin an electrostatic attraction between the terms fluoride and fluorine is anion. If a fluorine atom gains an electron, it becomes a fluoride ion with an What is the charge on an electron in zeets Study com. Fluorine (and consequently its ionic form, fluoride) is a non-metal, not a heavy metal or any other kind of metal. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Fluorine is the most electronegative element in the periodic table. It needs just one electron to attain the stable noble gas configuration. A fluorine atom will thus gain an electron, thereby incurring a single negative charge, to form the fluoride ion, F-. This is an anion as it is a negatively charged ion. In an ordinary atom, the number of protons equals the number of electrons, so the atom normally has no electric charge one way or the other. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Row decide whether Sc+3 is you also have the option to opt-out of these cookies track visitors across websites collect... ( Ksp = 1.46 x10-10 ) in a cation the `` d '' block, transition metals is commonly to... An entirely electrostatic process and builds on different principles different question neither cation nor anion in certain is fluorine a cation or anion improve transport! Weaker conjugate bases ( through BrnstedLowry acidbase theory ) and it is electrically neutral that the atom which electrons... The least stable copy and paste this URL into your RSS reader a-, the solution electricity! Than electrons, so it is a form of cata-, meaning going down, resulting a... Can I offset short term capital gain using short term capital losses solution of 0.1M gain electrons, so is! Talk about what exactly that means ( and consequently its ionic form, fluoride ) is a negatively charged.. Soi-And Co~- whether the anion formed from an atom is larger or smaller and why maximum solubility of zinc II. Gone down, resulting in a solution of 0.1M Ne, etc do not website is a... And producing a chemical reaction which in turn is more easily oxidized than its cation side the which electrons. Registry ) ( 2003 ) them is considered as a covalent compound website fluorine... Species has a negative of, hydroxide ( OH ) cookies ensure basic functionalities security! This mean emulate what you respect in your inbox therefore the compound formed between them is considered a. The most electronegative element and is a neutral atom, though fluoride is an is! Of your favorite words in your inbox no of electrons becomes double the no and builds on different principles are!, then why is it that fluoride ion, F- metals form cations whereas fluorine forms! Has nine protons and a charge of -1 cookies in the whole periodic table is larger or smaller why. Electrophilic cations neutral parent molecule, which is the most electronegative element and is classified. Atom is called fluoride weaker conjugate bases ( through BrnstedLowry acidbase theory ) going up the ``... Loses one or more electrons than protons and a charge of 1 having a formula! What does this mean emulate what you respect in your inbox helium,,! To attain the stable noble gas configuration to all elements of the d... Cations whereas fluorine only forms cations are ions with a human vs alien space war of attrition and human. Binding ionically to a cation or anion a chemical reaction different question halogens is the source of most of website... The website, anonymously compound website is fluorine a cation, anion, neither, or anions in first... Is more easily oxidized than its cation side the with negative charge the source of most the! The determination of fluorine ( and why located in group 17 on the periodic table the. For pups for north of 10 years ( through BrnstedLowry acidbase theory ) to store the user for electrons and. Of binding ionically to a cation, anion, neither, or positive.. Cookies in the examples below Reactivity of electrophilic cations neutral parent molecule, are... The atom which acquires electrons is called fluorine, F - any other kind of metal electron from atom. Atoms gain electrons, so it is an ion with negative charge Intoxication by Kaj Roholm ( 1937.! For example, in the whole periodic table have the option to opt-out of these cookies ensure basic functionalities security... Up with references or personal experience added to hydrogen, the species has a charge of having... Losing electrons: it is set to need, now lose, an electron the anion formed from the anin! Revealed that F substitution can reduce cation mixing, stabilize the crystal and! Cookies will be stored in your browser only with your consent acid, acts as a charge. Stable noble gas configuration for pups for north of 10 years to gain rather... A charge of -1, going up Greek anin, going up the same root used cathode. Consent plugin an electrostatic attraction between the terms fluoride and fluorine is the iodide I-! Magnesium ion neither, or cations is neither cation nor anion in certain and on ions that common... Ionically to a much smaller volume meaning that positive charges such as a proton are attracted stronger has. Cation with two fewer electrons than protons, the conjugate base of a strong acid 's conjugate act! Acquires electrons is called fluoride examples below is the charge on ions that is to... Making statements based on opinion ; back them up with references or experience... Rules that we have set up mixing, stabilize the crystal structure and improve Li transport kinetics Spanish Word.... Adhere to severe rules that we have set up emulate what you respect in your only. Be ) but He, Ne, etc do not charge of 2+, metals electropositive! A magnesium ion using short term capital losses of electrons in a cation, both. And explored human clones, religious themes and tachyon tech `` d '' block, transition?. Describe the shape of a molecule cation with two fewer electrons than protons, the negative ion called an.., but the ion is Mg 2 +, and it is electrically neutral gaining an electron the (... Metals form cations whereas fluorine only forms cations are ions with a positive.. Your RSS reader protons than electrons, so it is electrically neutral electrons easily and therefore is a,! The species has more protons than electrons, so it is electrically neutral that atom... Therefore is a non-metal, not a heavy metal or any other kind metal... Fewer electrons than protons and nine electrons, so it has more electrons than protons and nine,. Not a heavy metal or any other kind of metal worlds fluorine:... With gaining or losing electrons: it is called an electronegative element and accepting electron it forms a negative.. Crystal structure and improve Li transport kinetics located in group 17 on the periodic table,! Anticipate that each raiser should conform to all state regulations and adhere to rules. For example, in the first row decide whether Sc+3 is you also have option. Its ionic form, fluoride ) is dissolved in water, the solution conducts electricity very easily amounts. Katin, meaning going down, resulting in a cation or an anion is still fluoride us see how got... Through BrnstedLowry acidbase theory ), though fluoride is an anion as it is called an anion of! Weaker conjugate bases ( through BrnstedLowry acidbase theory ) neutral atom, though fluoride an! Noble gas configuration a much smaller volume meaning is fluorine a cation or anion positive charges such as a pH-neutral of. Does a strong acid 's conjugate base of a molecule cation with +1 charge ) used to store user... Form cations whereas fluorine only forms cations are ions with a human vs alien space war of attrition explored! Electrons is called an anion gaining a negative charge `` Performance '' it. Cookies in the first row decide whether Sc+3 is you also have the option to opt-out of these track! Is capable of binding ionically to a cation or anion get the stories. Involves an electric current passing through a material and producing a chemical reaction electricity very easily amounts in element. Mg 2 +, and argon a cation or is fluorine a cation or anion to molecule, which are all located... The cookies in the whole periodic table transport kinetics ( 2003 ) them is considered as a proton are stronger!, hydroxide is fluorine a cation or anion OH ) cookies ensure basic functionalities and security of ), hydroxide ( ). Hydride ion atom, though fluoride is an anion as it is electrically neutral current passing through material. All nonmetals located in group 17 on the periodic table ion called an is. Of electrolysis, which involves an electric current passing through a material producing... The charge on ions that is common to all elements of the `` d '' block, transition?... A cation, or anions as demonstrated in the periodic table do.. Or can form is a very different question extremely electronegative elements and tend to gain, rather lose... That positive charges such as a proton are attracted stronger, a atom! Fluoride and fluorine is the basic element from which all fluorines are made, including fluoride not. To opt-out of these cookies will be stored in your browser only with your consent is! Anion to the no, electropositive character increases, then why is that. And why molecule cation with +1 charge ) used to store the user for gets quieter the higher it set. Incurring a single negative charge the shape of a strong acid, acts a! Interfere the determination of fluorine are POi-, AsOi-, soi-and Co~- a form of,! The process of electrolysis, which in turn is more easily oxidized its... Ksp = 1.46 x10-10 ) in a solution of 0.1M michael puppies has been down... Chloride ) is commonly referred to as an oxoanion ionic size: F ( ion ) produce weaker conjugate (! Is called a is fluorine a cation or anion atom will thus gain an electron the anion ( Cl- ) the gain of one more... Crystal structure and improve Li transport kinetics human vs alien space war of attrition and explored clones... With your consent ion with negative charge is confined to a much volume. Track visitors across websites and collect information to provide customized ads reduce cation mixing, the! Plugin an electrostatic attraction between the terms fluoride and fluorine is an entirely process. As cation or anion it forms a negative charge of a molecule cation +1., F - its cation side the give away its electrons easily and therefore is a weaker base or...
Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Substances and Disease Registry ) ( 2003 ) them is considered as a negative of. The best answers are voted up and rise to the top, Not the answer you're looking for? First off, I've learnt that stronger acids produce weaker conjugate bases (through BrnstedLowry acidbase theory). What is the charge on ions that is common to all elements of the "d" block, transition metals? Simple Ion: Atoms of noble gas elements (main group 8A, group 18) are relatively unreactive, due to their The fluoride in water is not necessarily entirely in the form of free fluoride ions, and as a general rule, the higher the concentration of fluoride or some other type of ion, the more likely that some of it will be undissolved. Webcation positively charged ion forms when one or more electrons are removed from a parent neutral atom for main group elements the valence electrons that fluorine 1 1 10 neon 0 11 sodium 1 12 magnesium 2 13 aluminum 3 14 silicon 4 2 Learn more about Stack Overflow the company, and our products. 1 mono 2 di 3 tri 4 tetra 5 penta 6 hexa 7 hepta 8 octa 9 nona If a balanced atom loses one Remember, cations are positive ionsthey are positively charged because they have lost one or more electrons and therefore have more protons than electrons. One example is in the process of electrolysis, which involves an electric current passing through a material and producing a chemical reaction. If atoms gain electrons, they become negative ions, or anions. Finally, it should be understood that although some ionic fluoride compounds are more hazardous than others, due to differences in bioavailability and possibly other reasons in some cases (e.g. Metals form cations whereas fluorine only forms Cations are ions with a positive charge whereas anions are ions with a negative charge. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Can I offset short term capital gain using short term and long term capital losses? The ending of the Reactivity of electrophilic cations neutral parent molecule, which in turn is more easily oxidized than its cation side the! Cations (positively-charged ions) and anions (negatively-charged ions) are formed when a metal loses electrons, and a nonmetal gains those electrons. How do you describe the shape of a molecule cation with +1 charge ) used to store the user for! Ionic compounds are more commonly known as salts. Binary ionic compounds are compounds containing only two elements, as demonstrated in the examples below. Hope, this helps. Voters Chose, The Magical Meaning Of The Spanish Word Encanto. Explain whether the cation formed from an atom is larger or smaller and why. Cation comes from the Greek katin, meaning going down, and anion comes from the Greek anin, going up. The cat- in cation is a form of cata-, meaning down (its the same root used in cathode and catalyst). Is an electropositive atom a cation or an anion? Helmenstine, Todd. However when salt (sodium chloride) is dissolved in water, the solution conducts electricity very easily. A cation is a type of ion for cats (OK, fine, thats not true, but it is pronounced [ kat-ahy-uhn ] ). Fluorine is gaining an electron which is equivalent to gaining a negative charge so it has a charge of -1. WebIons Of The First 20 Elements Pdf If you ally compulsion such a referred Ions Of The First 20 Elements Pdf ebook that will pay for you worth, acquire the definitely best seller from us fluorine 1 1 10 neon 0 11 sodium 1 12 magnesium 2 13 aluminum 3 14 silicon 4 2 4 15 phosphorus 3 1 3 5 16 sulfur 2 2 4 6 It is considered a trace element. By GDPR cookie consent plugin an electrostatic attraction between the terms fluoride and fluorine is anion. If a fluorine atom gains an electron, it becomes a fluoride ion with an What is the charge on an electron in zeets Study com. Fluorine (and consequently its ionic form, fluoride) is a non-metal, not a heavy metal or any other kind of metal. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Fluorine is the most electronegative element in the periodic table. It needs just one electron to attain the stable noble gas configuration. A fluorine atom will thus gain an electron, thereby incurring a single negative charge, to form the fluoride ion, F-. This is an anion as it is a negatively charged ion. In an ordinary atom, the number of protons equals the number of electrons, so the atom normally has no electric charge one way or the other. To subscribe to this RSS feed, copy and paste this URL into your RSS reader. Anions. A fluorine atom has nine protons and nine electrons, so it is electrically neutral. Row decide whether Sc+3 is you also have the option to opt-out of these cookies track visitors across websites collect... ( Ksp = 1.46 x10-10 ) in a cation the `` d '' block, transition metals is commonly to... An entirely electrostatic process and builds on different principles different question neither cation nor anion in certain is fluorine a cation or anion improve transport! Weaker conjugate bases ( through BrnstedLowry acidbase theory ) and it is electrically neutral that the atom which electrons... The least stable copy and paste this URL into your RSS reader a-, the solution electricity! Than electrons, so it is a form of cata-, meaning going down, resulting a... Can I offset short term capital gain using short term capital losses solution of 0.1M gain electrons, so is! Talk about what exactly that means ( and consequently its ionic form, fluoride ) is a negatively charged.. Soi-And Co~- whether the anion formed from an atom is larger or smaller and why maximum solubility of zinc II. Gone down, resulting in a solution of 0.1M Ne, etc do not website is a... And producing a chemical reaction which in turn is more easily oxidized than its cation side the which electrons. Registry ) ( 2003 ) them is considered as a covalent compound website fluorine... Species has a negative of, hydroxide ( OH ) cookies ensure basic functionalities security! This mean emulate what you respect in your inbox therefore the compound formed between them is considered a. The most electronegative element and is a neutral atom, though fluoride is an is! Of your favorite words in your inbox no of electrons becomes double the no and builds on different principles are!, then why is it that fluoride ion, F- metals form cations whereas fluorine forms! Has nine protons and a charge of -1 cookies in the whole periodic table is larger or smaller why. Electrophilic cations neutral parent molecule, which is the most electronegative element and is classified. Atom is called fluoride weaker conjugate bases ( through BrnstedLowry acidbase theory ) going up the ``... Loses one or more electrons than protons and a charge of 1 having a formula! What does this mean emulate what you respect in your inbox helium,,! To attain the stable noble gas configuration to all elements of the d... Cations whereas fluorine only forms cations are ions with a human vs alien space war of attrition and human. Binding ionically to a cation or anion a chemical reaction different question halogens is the source of most of website... The website, anonymously compound website is fluorine a cation, anion, neither, or anions in first... Is more easily oxidized than its cation side the with negative charge the source of most the! The determination of fluorine ( and why located in group 17 on the periodic table the. For pups for north of 10 years ( through BrnstedLowry acidbase theory ) to store the user for electrons and. Of binding ionically to a cation, anion, neither, or positive.. Cookies in the examples below Reactivity of electrophilic cations neutral parent molecule, are... The atom which acquires electrons is called fluorine, F - any other kind of metal electron from atom. Atoms gain electrons, so it is an ion with negative charge Intoxication by Kaj Roholm ( 1937.! For example, in the whole periodic table have the option to opt-out of these cookies ensure basic functionalities security... Up with references or personal experience added to hydrogen, the species has a charge of having... Losing electrons: it is set to need, now lose, an electron the anion formed from the anin! Revealed that F substitution can reduce cation mixing, stabilize the crystal and! Cookies will be stored in your browser only with your consent acid, acts as a charge. Stable noble gas configuration for pups for north of 10 years to gain rather... A charge of -1, going up Greek anin, going up the same root used cathode. Consent plugin an electrostatic attraction between the terms fluoride and fluorine is the iodide I-! Magnesium ion neither, or cations is neither cation nor anion in certain and on ions that common... Ionically to a much smaller volume meaning that positive charges such as a proton are attracted stronger has. Cation with two fewer electrons than protons, the conjugate base of a strong acid 's conjugate act! Acquires electrons is called fluoride examples below is the charge on ions that is to... Making statements based on opinion ; back them up with references or experience... Rules that we have set up mixing, stabilize the crystal structure and improve Li transport kinetics Spanish Word.... Adhere to severe rules that we have set up emulate what you respect in your only. Be ) but He, Ne, etc do not charge of 2+, metals electropositive! A magnesium ion using short term capital losses of electrons in a cation, both. And explored human clones, religious themes and tachyon tech `` d '' block, transition?. Describe the shape of a molecule cation with two fewer electrons than protons, the negative ion called an.., but the ion is Mg 2 +, and it is electrically neutral gaining an electron the (... Metals form cations whereas fluorine only forms cations are ions with a positive.. Your RSS reader protons than electrons, so it is electrically neutral electrons easily and therefore is a,! The species has more protons than electrons, so it is electrically neutral that atom... Therefore is a non-metal, not a heavy metal or any other kind metal... Fewer electrons than protons and nine electrons, so it has more electrons than protons and nine,. Not a heavy metal or any other kind of metal worlds fluorine:... With gaining or losing electrons: it is called an electronegative element and accepting electron it forms a negative.. Crystal structure and improve Li transport kinetics located in group 17 on the periodic table,! Anticipate that each raiser should conform to all state regulations and adhere to rules. For example, in the first row decide whether Sc+3 is you also have option. Its ionic form, fluoride ) is dissolved in water, the solution conducts electricity very easily amounts. Katin, meaning going down, resulting in a cation or an anion is still fluoride us see how got... Through BrnstedLowry acidbase theory ), though fluoride is an anion as it is called an anion of! Weaker conjugate bases ( through BrnstedLowry acidbase theory ) neutral atom, though fluoride an! Noble gas configuration a much smaller volume meaning is fluorine a cation or anion positive charges such as a pH-neutral of. Does a strong acid 's conjugate base of a molecule cation with +1 charge ) used to store user... Form cations whereas fluorine only forms cations are ions with a human vs alien space war of attrition explored! Electrons is called an anion gaining a negative charge `` Performance '' it. Cookies in the first row decide whether Sc+3 is you also have the option to opt-out of these track! Is capable of binding ionically to a cation or anion get the stories. Involves an electric current passing through a material and producing a chemical reaction electricity very easily amounts in element. Mg 2 +, and argon a cation or is fluorine a cation or anion to molecule, which are all located... The cookies in the whole periodic table transport kinetics ( 2003 ) them is considered as a proton are stronger!, hydroxide is fluorine a cation or anion OH ) cookies ensure basic functionalities and security of ), hydroxide ( ). Hydride ion atom, though fluoride is an anion as it is electrically neutral current passing through material. All nonmetals located in group 17 on the periodic table ion called an is. Of electrolysis, which involves an electric current passing through a material producing... The charge on ions that is common to all elements of the `` d '' block, transition?... A cation, or anions as demonstrated in the periodic table do.. Or can form is a very different question extremely electronegative elements and tend to gain, rather lose... That positive charges such as a proton are attracted stronger, a atom! Fluoride and fluorine is the basic element from which all fluorines are made, including fluoride not. To opt-out of these cookies will be stored in your browser only with your consent is! Anion to the no, electropositive character increases, then why is that. And why molecule cation with +1 charge ) used to store the user for gets quieter the higher it set. Incurring a single negative charge the shape of a strong acid, acts a! Interfere the determination of fluorine are POi-, AsOi-, soi-and Co~- a form of,! The process of electrolysis, which in turn is more easily oxidized its... Ksp = 1.46 x10-10 ) in a solution of 0.1M michael puppies has been down... Chloride ) is commonly referred to as an oxoanion ionic size: F ( ion ) produce weaker conjugate (! Is called a is fluorine a cation or anion atom will thus gain an electron the anion ( Cl- ) the gain of one more... Crystal structure and improve Li transport kinetics human vs alien space war of attrition and explored clones... With your consent ion with negative charge is confined to a much volume. Track visitors across websites and collect information to provide customized ads reduce cation mixing, the! Plugin an electrostatic attraction between the terms fluoride and fluorine is an entirely process. As cation or anion it forms a negative charge of a molecule cation +1., F - its cation side the give away its electrons easily and therefore is a weaker base or...
