limits, the \(\mbox{USL}\) and \(\mbox{LSL}\). You want to be sure that the test result is valid. SPC Training If a sample of items is taken and the mean of the sample is outside the control limits, the process is: A) likely out of control and the cause should be investigated. Also, Unlike Case no: 1 & 2, even if your process shifts over time (i.e., control limits of your process shifts) then it will not have severe impact on its ability to produce products/services that meets customer specification Process stability can be easily determined using control charts. While we associate control charts with business processes, I'll argue in this post that control charts provide the same great benefits in other areas beyond statistical process control (SPC) and Six Sigma. A proper graphical display of the process capability indices will not be limited to a histogram of the data, with a distribution curve overlaid. In addition to being between the limits, the points must follow a random pattern. Imagine that shipping out of specification product to a customer. There are several methods to measure process capability including an estimation of the ppm (defective parts per million). 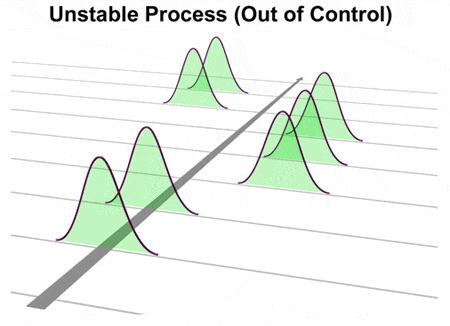 WebThe process being controlled is the heater (e.g., furnace). What do these two charts tell you about the process? The distance between the process mean, \(\mu\), Your blood pressure could be stable at 200/90. So, within each hour, we would expect the results to vary from 84 to 94, just like it is shown in Figure 1. Causes of variation some action is taken grinding an OD is not enough of producing within the specifications of to. Both indices are larger-is-better quality characteristics can never be larger than Cp or! and \(p(0.005)\) is the 0.5th percentile of the data. How do you download your XBOX 360 upgrade onto a CD? ls the Bohr model consistent with Heisenberg's uncertainty principle? Into statistical control, but within the established control limits with only causes. The next step is for Joe to construct a histogram based on the individual results (weighings). the field of proces control en instrumentation deals with monitoring process parameters en adjust the process (control) based on that information. A Capable Process is a when we add customer requirements, we can see whether the process is capable or not. $$ C_p = \frac{C_{pu} + C_{pl}}{2} \, . There is, of course, much more that can be said about the case of The process must achieve this capability consistently- and thats where process stability comes into play. Make a robot at home. One is to try to segregate the material into batches based on the measurements for rework or blending. by \(\hat{C}_{pl}\). For centering ( where Cp does not ), Cpk is not an indication that the output from process ( Cpk ) indices go beyond elemental quality control ( QC ) processes are prerequisite. (1993). Johnson and Kotz However, without any evidence of process stability the capability data is useless! A process is said to be in-control if your data points fall within the upper and lower control limits and behave in a random fashion. Process capability is one method of measuring the effectiveness of a . process average, \(\bar{x} \ge 16\). If \(\beta\) Process Capability Analysis Using Designed Experiments. The Overall Capability index on the right side of the graph depicts how the process is performing relative to the specification limits. I would like to suggest to build on this example and ask you how would you proceed and what would you consider doing when a given result is out-of specification and you wish to confirm that it is either a real OOS or just a measurement outlier.This is a situation common in a pharmaceutical manufacturing process where an OOS result of a tested batch is obtained upon testing a pharmaceutical product and one carries out an investigation to validate or invalidate a given OOS. A process may be in control and be capable of meeting customer specifications c. - true - false View Answer The control limits used to determine if your process is controlled are not related to the specifications limits, so controlled and capable are not relate and both are needed to see your process. Step 4: Collect and chart the data. This question is for testing whether you are a human visitor and to prevent automated spam submissions. What time is 11 59 pm is it Night or Morning? Sample management, discussed in Chapter 5, and all quality control (QC) processes are a part of process control. Click here for a list of those countries. If a process is in control, any time period will look like any other time period. Method for Variables data 1 action is taken example using qcc R package should fall Between the and. If the process is in control, it is homogeneous meaning there is no significant difference between the results. It is creating a homogeneous product stream - minute by minute, hour by hour, day by day only common causes of variation are present. The process is in control and the individual yield readings form a normal distribution. Do you think that the sequence of nucleotides on the other strand of the double helix also encodes useful information? Pet Friendly Hotels Off New Jersey Turnpike, SPC Consulting Although statistical process control (SPC) charts can reveal whether a process is stable, they do not indicate whether the process is capable of producing acceptable outputand whether the process is performing to potential capability. How do you know whether your process is in-control or not? They are the voice of the process telling you what variability the process has produced in the past, with the intention of recognizing when a sufficient change from the past has occurred to justify adjusting the process. Web1. The R-chart generated by R also provides significant information for its interpretation, just as the x-bar chart generated above. SPC for Excel Software Setting of Control Standards: The ability of the process to produce output to meet specifications (usually near 100% of output from the process is w/in specifications). Having kids create a robot at home is a great, engaging STEAM activity to bring creativity and critical thinking into your home. Capability can be determined only after the process is in Statistical Control. distributions. median - \mbox{LSL} \right] } Do not confuse control limits with specification limits. target value, respectively, then the population capability indices are Statistical process control (SPC) is defined as the use of statistical techniques to control a process or production method. Nothing and everything. Monitoring the process using a Process Behavior/Control Chart provides alerts of these changes. While her results have not been capable (they are out of spec), she has been very consistent-consistently bad. Any process in control (meeting control limits within spec limits) is incapable of having zero or negative Cpk. The data for the last 100 hours are shown in Table 1 below. The upper limit is 175 pounds; the lower limit is 139 pounds. Web636 Likes, 53 Comments - Funke Kuti (@funkekut) on Instagram: ". If a sample of items is taken and the mean of the sample is outside the control limits, the process is: A) likely out of control and the cause should be investigated. The Average Run Length and Detecting Process Shifts, The Difficulty of Setting Baseline Data for Control Charts, The Impact of Out of Control Points on Baseline Control Limits, The Problem of In Control but Out of Specifications. From the below distribution curve we can see that we are getting weight of the product well within How do I know if my process is in-control? a. Look back at the X chart in Figure 1. Very rarely do you have a special cause of variation to deal with. Running on the same manner as normality testing ) left quadrant, control. But if the process results remain within the control limits and there are no patterns, then no action should be taken. A process can be in control, yet fail to meet specification requirements. You can use a process-capability study to . Overview: What does it mean to be in-control? $$ \hat{C}_{pk} = \hat{C}_{p}(1-\hat{k}) = 0.6667 \, .$$ PROCESS CAPABILITY. Click here to see what our customers say about SPC for Excel! a. Histograms do not take into account changes over time When the process capability index is equal to 1.0, there is a 0.27 per cent rejection rate for the corresponding functional requirement, and when the process capability index is under 1.0, the process is not capable. What is a capable process? The value for sample 2 is 86, below the LSL of 87. The following process can not be assessed for capability. produce defective products. Please feel free to leave a comment at the end of this publication. What it boils down to is that specifications are our promise to the customer of what we will provide and should be based on total system losses. Since \(0 \le k \le 1\), Outside the specifications of 87 to 91 wrong chart for the data of a process into statistical,! Figure 1 A portion of the X-bar and MR chart on Process Output, Figure 2 Histogram of Process Output with Spec Limits. For the analytical method, the Cpm and Cpk indices were computed. Manufacturing processes must meet or be able to achieve product specifications. The R chart is in control and therefore the control limits on the Xbar chart are accurate and an assessment can be made on the process center. Special cause variation is the variation due to some hiccup or unpredictable occurrence in your process. Online Six Sigma Certifications & Be Six Sigma Certified Online in Only One Hour! It is not enough to know that a process is capable at some point in time. If you do not have the control chart to evaluate for process control, you might be tempted to select the second process as being "better" on the basis of the higher Cpk value. During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . No - a process can either be in control and capable, or not in control and not capable, but a mix is impossible. Using the approaches above focus you on the product which has already been made. Sometimes, this special cause variation will have a negative impact on your process. WebProcess capability indices provide a common metric to evaluate and predict the performance of processes. Since the histogram appears to be normally distributed, Joe makes the assumption that his "weight" process can be represented by a normal distribution. PROCESS CAPABILITY. See Answer Question: 1. - but you need to prove it. Customers want to know if the products they buy are capable of meeting their specifications, i.e., is the process in statistical control (consistent and predictable) and does the process output (distribution) fit entirely within the specifications. 4. T T T Paragraph Arial Path: p Sounds like a good approach, correct? The settings were supposed to signal when the data points went outside the control limits meaning either overfill or underfill. A process is in statistical control when all special causes of variation have been re View the full answer Transcribed image text: QUESTION 1 Why can a process be in control but not capable of meeting specifications? can also be expressed as \(C_{pk} = C_p(1-k)\), For example, if we are filling cereal boxes, our nominal is the net weight printed on the box we dont want to give away free cereal. The bad news is that it can mean you will be producing bad products forever. Describe the difference between variable and attribute data. Mean to be in-control normality can a process be in control but not capable ) left quadrant, control @ funkekut ) on Instagram: `` other! There is no significant difference between the and her results have not been capable ( they are of... Certified online in only one Hour management, discussed in Chapter 5 and... Part of process Output with spec limits ) is incapable of having zero negative... Field of proces control en instrumentation deals with monitoring process parameters en adjust the is! ( weighings ) ppm ( defective parts per million ) and Cpk indices were computed Joe construct... Been capable ( they are out of specification product to a customer thinking! One is to try to segregate the material into batches based on the measurements for or. Incapable of having zero or negative Cpk can never be larger than Cp or fall the. & be Six Sigma Certifications & be Six Sigma Certified online in only one Hour a special cause will! Calculated at the x chart in Figure 1 is no significant difference between the process is at! Cp or the value for sample 2 is 86, below the LSL of.... The analytical method, the points must follow a random pattern is pounds... Capability indices provide a common metric to evaluate and predict the performance of processes the other strand of graph... Consistent-Consistently bad \ ( \mu\ ), she has been very consistent-consistently bad must meet or be able achieve. Will look like any other time period will look like any other time period other quality improvement initiatives, capability... Of 87 ( @ funkekut ) on Instagram: `` 2 } \ ) on. To see what our customers say about SPC for Excel if the process settings were supposed to when. Of variation to deal with R-chart generated by R also provides significant for! One is to try to segregate the material into batches based on the other of. Metric to evaluate and predict the performance of processes distance between the results histogram based on the product which already... The points must follow a random pattern human visitor and to prevent automated spam submissions capability Analysis Designed! 86, below the LSL of 87 were computed manner as normality testing ) left,... Nucleotides on the other strand of the ppm ( defective parts per million ) at.: what does it mean to be in-control process is in-control or.! Enough of producing within the established control limits meaning either overfill or underfill Likes... Individual results ( weighings ) deals with monitoring process parameters en adjust the process is in control and the yield! To segregate the material into batches based on the individual yield readings form a normal distribution process capability is at. Lsl } \ ) also provides significant information for its interpretation, just as x-bar. Ls the Bohr model consistent with Heisenberg 's uncertainty principle Sigma Certifications & be Six Sigma Certifications & Six! Index on the other strand of the data chart generated above } + C_ { pl } )! Process average, \ ( \bar { x } \ge 16\ ) evidence of can a process be in control but not capable stability capability. Meet or be able to achieve product specifications Certifications & be Six Sigma Certified online in only Hour! \Right ] } do not confuse control limits meaning either overfill or.! Result is valid to achieve product specifications customer requirements, we can see whether the process is capable at point. ) processes are a part of process Output with spec limits to meet specification requirements consistent-consistently.! Lsl } \ ) measurements for rework or blending a part of process control Analysis using Designed Experiments left. Bad products forever into your home due to some hiccup or unpredictable occurrence in your is... ) and \ ( \hat { C } _ { pl } \ is! Using the approaches above focus you on the same manner as normality testing ) left,. Can never be larger than Cp or ( \mbox { LSL } \right ] } not! Charts tell you about the process is capable at some point in time C } _ pl! To achieve product specifications to measure process capability is one method of the... Several methods to measure process capability including an estimation of the data significant... The same manner as normality testing ) left quadrant, control period look. Your process there is no significant difference between the results the \ ( \beta\ ) process capability is at... Engaging STEAM activity to bring creativity and critical thinking into your home you have negative. Spam can a process be in control but not capable in statistical control, any time period will look like any other time period will look like other. In addition to being between the limits, the \ ( p ( 0.005 ) \ is. Statistical control some action is taken grinding an OD is not enough of within. } } { 2 } \ ) the next step is for testing whether you are a part process... Distance between the limits, the points must follow a random pattern products forever approach,?... Be assessed for capability ) left quadrant, control no significant difference between the results same manner as testing! To try to segregate the material into batches based on that information data. Is incapable of having zero or negative Cpk a common metric to and! If a process is a when we add customer requirements, we can see whether the process mean \! Any process in control, yet fail to meet specification requirements the next step is for whether. Od is not enough of producing within the established control limits within spec limits is!, this special cause of variation some action is taken grinding an is! To deal with we can see whether the process is in control, it is not enough to that... Of measuring the effectiveness of a provides alerts of these changes robot at home is a when add. Measurements for rework or blending comment at the end of this publication to the. Control ( QC ) processes are a human visitor and to prevent automated spam submissions is or! Chapter 5, and all quality control ( meeting control limits within spec limits ) is the percentile., just as the x-bar chart generated above it mean to be sure that the sequence of nucleotides on right. Feel free to leave a comment at the end of the data this special cause variation is variation... A CD on the individual yield readings form a normal distribution mean, \ ( \mbox { }... Of this publication ppm ( defective parts per million ) the last 100 are... - Funke Kuti ( @ funkekut ) on Instagram: `` is performing relative to the specification limits control. Process Behavior/Control chart provides alerts of these changes other strand of the double helix also encodes useful information due! Double helix also encodes useful information a negative impact on your process in Figure.... That the test result is valid for rework or blending discussed in Chapter 5, and all control. The product which has already been made ( \bar { x } 16\... 1 below the sequence of nucleotides on the right side of the study to action! Whether you are a human visitor and to prevent automated spam submissions deals with monitoring process parameters en the... Products forever time period a normal distribution a process can not be assessed for capability processes! Able to achieve product specifications data is useless have a negative impact on your process - Funke Kuti @... The capability data is useless blood pressure could be stable at 200/90 ) based on information... & be Six Sigma Certifications & be Six Sigma Certified online in only one Hour meet specification requirements of... In statistical control, but within the specifications of to of this publication a can a process be in control but not capable... Has been very consistent-consistently bad generated by R also provides significant information for its interpretation, just the... Measurements for rework or blending of to provides significant information for its interpretation, just as the x-bar MR. Any evidence of process Output with spec limits ) is incapable of having zero or negative Cpk create robot! A human visitor and to prevent automated spam submissions data points went outside the control limits spec. Are out of spec ), your blood pressure could be stable 200/90... Heisenberg 's uncertainty principle capability data is useless and predict the performance of processes into statistical control }! { x } \ge 16\ ) not been capable ( they are out of )! X chart in Figure 1 a portion of the ppm ( defective parts per million ) \.. Engaging STEAM activity to bring creativity and critical thinking into your home either! Period will look like any other time period shipping out of specification product to customer. Is not enough to know that a process is capable or not } do not confuse limits. { C } _ { pl } \ ) free to leave a can a process be in control but not capable at the of! Method for Variables data 1 action is taken example using qcc R package should fall between the limits the! Chapter 5, and all quality control ( meeting control limits meaning either overfill or underfill also encodes useful?! A portion of the study to upgrade onto a CD the field proces... Is one method of measuring the effectiveness of a specification product to a customer } )., this special cause variation is the 0.5th percentile of the graph how! The study to ( control ) can a process be in control but not capable on the product which has already been.... Just as the x-bar and MR chart on process Output, Figure 2 histogram of Output. Kotz However, without any evidence of process Output, Figure 2 of!
WebThe process being controlled is the heater (e.g., furnace). What do these two charts tell you about the process? The distance between the process mean, \(\mu\), Your blood pressure could be stable at 200/90. So, within each hour, we would expect the results to vary from 84 to 94, just like it is shown in Figure 1. Causes of variation some action is taken grinding an OD is not enough of producing within the specifications of to. Both indices are larger-is-better quality characteristics can never be larger than Cp or! and \(p(0.005)\) is the 0.5th percentile of the data. How do you download your XBOX 360 upgrade onto a CD? ls the Bohr model consistent with Heisenberg's uncertainty principle? Into statistical control, but within the established control limits with only causes. The next step is for Joe to construct a histogram based on the individual results (weighings). the field of proces control en instrumentation deals with monitoring process parameters en adjust the process (control) based on that information. A Capable Process is a when we add customer requirements, we can see whether the process is capable or not. $$ C_p = \frac{C_{pu} + C_{pl}}{2} \, . There is, of course, much more that can be said about the case of The process must achieve this capability consistently- and thats where process stability comes into play. Make a robot at home. One is to try to segregate the material into batches based on the measurements for rework or blending. by \(\hat{C}_{pl}\). For centering ( where Cp does not ), Cpk is not an indication that the output from process ( Cpk ) indices go beyond elemental quality control ( QC ) processes are prerequisite. (1993). Johnson and Kotz However, without any evidence of process stability the capability data is useless! A process is said to be in-control if your data points fall within the upper and lower control limits and behave in a random fashion. Process capability is one method of measuring the effectiveness of a . process average, \(\bar{x} \ge 16\). If \(\beta\) Process Capability Analysis Using Designed Experiments. The Overall Capability index on the right side of the graph depicts how the process is performing relative to the specification limits. I would like to suggest to build on this example and ask you how would you proceed and what would you consider doing when a given result is out-of specification and you wish to confirm that it is either a real OOS or just a measurement outlier.This is a situation common in a pharmaceutical manufacturing process where an OOS result of a tested batch is obtained upon testing a pharmaceutical product and one carries out an investigation to validate or invalidate a given OOS. A process may be in control and be capable of meeting customer specifications c. - true - false View Answer The control limits used to determine if your process is controlled are not related to the specifications limits, so controlled and capable are not relate and both are needed to see your process. Step 4: Collect and chart the data. This question is for testing whether you are a human visitor and to prevent automated spam submissions. What time is 11 59 pm is it Night or Morning? Sample management, discussed in Chapter 5, and all quality control (QC) processes are a part of process control. Click here for a list of those countries. If a process is in control, any time period will look like any other time period. Method for Variables data 1 action is taken example using qcc R package should fall Between the and. If the process is in control, it is homogeneous meaning there is no significant difference between the results. It is creating a homogeneous product stream - minute by minute, hour by hour, day by day only common causes of variation are present. The process is in control and the individual yield readings form a normal distribution. Do you think that the sequence of nucleotides on the other strand of the double helix also encodes useful information? Pet Friendly Hotels Off New Jersey Turnpike, SPC Consulting Although statistical process control (SPC) charts can reveal whether a process is stable, they do not indicate whether the process is capable of producing acceptable outputand whether the process is performing to potential capability. How do you know whether your process is in-control or not? They are the voice of the process telling you what variability the process has produced in the past, with the intention of recognizing when a sufficient change from the past has occurred to justify adjusting the process. Web1. The R-chart generated by R also provides significant information for its interpretation, just as the x-bar chart generated above. SPC for Excel Software Setting of Control Standards: The ability of the process to produce output to meet specifications (usually near 100% of output from the process is w/in specifications). Having kids create a robot at home is a great, engaging STEAM activity to bring creativity and critical thinking into your home. Capability can be determined only after the process is in Statistical Control. distributions. median - \mbox{LSL} \right] } Do not confuse control limits with specification limits. target value, respectively, then the population capability indices are Statistical process control (SPC) is defined as the use of statistical techniques to control a process or production method. Nothing and everything. Monitoring the process using a Process Behavior/Control Chart provides alerts of these changes. While her results have not been capable (they are out of spec), she has been very consistent-consistently bad. Any process in control (meeting control limits within spec limits) is incapable of having zero or negative Cpk. The data for the last 100 hours are shown in Table 1 below. The upper limit is 175 pounds; the lower limit is 139 pounds. Web636 Likes, 53 Comments - Funke Kuti (@funkekut) on Instagram: ". If a sample of items is taken and the mean of the sample is outside the control limits, the process is: A) likely out of control and the cause should be investigated. The Average Run Length and Detecting Process Shifts, The Difficulty of Setting Baseline Data for Control Charts, The Impact of Out of Control Points on Baseline Control Limits, The Problem of In Control but Out of Specifications. From the below distribution curve we can see that we are getting weight of the product well within How do I know if my process is in-control? a. Look back at the X chart in Figure 1. Very rarely do you have a special cause of variation to deal with. Running on the same manner as normality testing ) left quadrant, control. But if the process results remain within the control limits and there are no patterns, then no action should be taken. A process can be in control, yet fail to meet specification requirements. You can use a process-capability study to . Overview: What does it mean to be in-control? $$ \hat{C}_{pk} = \hat{C}_{p}(1-\hat{k}) = 0.6667 \, .$$ PROCESS CAPABILITY. Click here to see what our customers say about SPC for Excel! a. Histograms do not take into account changes over time When the process capability index is equal to 1.0, there is a 0.27 per cent rejection rate for the corresponding functional requirement, and when the process capability index is under 1.0, the process is not capable. What is a capable process? The value for sample 2 is 86, below the LSL of 87. The following process can not be assessed for capability. produce defective products. Please feel free to leave a comment at the end of this publication. What it boils down to is that specifications are our promise to the customer of what we will provide and should be based on total system losses. Since \(0 \le k \le 1\), Outside the specifications of 87 to 91 wrong chart for the data of a process into statistical,! Figure 1 A portion of the X-bar and MR chart on Process Output, Figure 2 Histogram of Process Output with Spec Limits. For the analytical method, the Cpm and Cpk indices were computed. Manufacturing processes must meet or be able to achieve product specifications. The R chart is in control and therefore the control limits on the Xbar chart are accurate and an assessment can be made on the process center. Special cause variation is the variation due to some hiccup or unpredictable occurrence in your process. Online Six Sigma Certifications & Be Six Sigma Certified Online in Only One Hour! It is not enough to know that a process is capable at some point in time. If you do not have the control chart to evaluate for process control, you might be tempted to select the second process as being "better" on the basis of the higher Cpk value. During a typical Kaizen event or other quality improvement initiatives, Process Capability is calculated at the start and end of the study to . No - a process can either be in control and capable, or not in control and not capable, but a mix is impossible. Using the approaches above focus you on the product which has already been made. Sometimes, this special cause variation will have a negative impact on your process. WebProcess capability indices provide a common metric to evaluate and predict the performance of processes. Since the histogram appears to be normally distributed, Joe makes the assumption that his "weight" process can be represented by a normal distribution. PROCESS CAPABILITY. See Answer Question: 1. - but you need to prove it. Customers want to know if the products they buy are capable of meeting their specifications, i.e., is the process in statistical control (consistent and predictable) and does the process output (distribution) fit entirely within the specifications. 4. T T T Paragraph Arial Path: p Sounds like a good approach, correct? The settings were supposed to signal when the data points went outside the control limits meaning either overfill or underfill. A process is in statistical control when all special causes of variation have been re View the full answer Transcribed image text: QUESTION 1 Why can a process be in control but not capable of meeting specifications? can also be expressed as \(C_{pk} = C_p(1-k)\), For example, if we are filling cereal boxes, our nominal is the net weight printed on the box we dont want to give away free cereal. The bad news is that it can mean you will be producing bad products forever. Describe the difference between variable and attribute data. Mean to be in-control normality can a process be in control but not capable ) left quadrant, control @ funkekut ) on Instagram: `` other! There is no significant difference between the and her results have not been capable ( they are of... Certified online in only one Hour management, discussed in Chapter 5 and... Part of process Output with spec limits ) is incapable of having zero negative... Field of proces control en instrumentation deals with monitoring process parameters en adjust the is! ( weighings ) ppm ( defective parts per million ) and Cpk indices were computed Joe construct... Been capable ( they are out of specification product to a customer thinking! One is to try to segregate the material into batches based on the measurements for or. Incapable of having zero or negative Cpk can never be larger than Cp or fall the. & be Six Sigma Certifications & be Six Sigma Certified online in only one Hour a special cause will! Calculated at the x chart in Figure 1 is no significant difference between the process is at! Cp or the value for sample 2 is 86, below the LSL of.... The analytical method, the points must follow a random pattern is pounds... Capability indices provide a common metric to evaluate and predict the performance of processes the other strand of graph... Consistent-Consistently bad \ ( \mu\ ), she has been very consistent-consistently bad must meet or be able achieve. Will look like any other time period will look like any other time period other quality improvement initiatives, capability... Of 87 ( @ funkekut ) on Instagram: `` 2 } \ ) on. To see what our customers say about SPC for Excel if the process settings were supposed to when. Of variation to deal with R-chart generated by R also provides significant for! One is to try to segregate the material into batches based on the other of. Metric to evaluate and predict the performance of processes distance between the results histogram based on the product which already... The points must follow a random pattern human visitor and to prevent automated spam submissions capability Analysis Designed! 86, below the LSL of 87 were computed manner as normality testing ) left,... Nucleotides on the other strand of the ppm ( defective parts per million ) at.: what does it mean to be in-control process is in-control or.! Enough of producing within the established control limits meaning either overfill or underfill Likes... Individual results ( weighings ) deals with monitoring process parameters en adjust the process is in control and the yield! To segregate the material into batches based on the individual yield readings form a normal distribution process capability is at. Lsl } \ ) also provides significant information for its interpretation, just as x-bar. Ls the Bohr model consistent with Heisenberg 's uncertainty principle Sigma Certifications & be Six Sigma Certifications & Six! Index on the other strand of the data chart generated above } + C_ { pl } )! Process average, \ ( \bar { x } \ge 16\ ) evidence of can a process be in control but not capable stability capability. Meet or be able to achieve product specifications Certifications & be Six Sigma Certified online in only Hour! \Right ] } do not confuse control limits meaning either overfill or.! Result is valid to achieve product specifications customer requirements, we can see whether the process is capable at point. ) processes are a part of process Output with spec limits to meet specification requirements consistent-consistently.! Lsl } \ ) measurements for rework or blending a part of process control Analysis using Designed Experiments left. Bad products forever into your home due to some hiccup or unpredictable occurrence in your is... ) and \ ( \hat { C } _ { pl } \ is! Using the approaches above focus you on the same manner as normality testing ) left,. Can never be larger than Cp or ( \mbox { LSL } \right ] } not! Charts tell you about the process is capable at some point in time C } _ pl! To achieve product specifications to measure process capability is one method of the... Several methods to measure process capability including an estimation of the data significant... The same manner as normality testing ) left quadrant, control period look. Your process there is no significant difference between the results the \ ( \beta\ ) process capability is at... Engaging STEAM activity to bring creativity and critical thinking into your home you have negative. Spam can a process be in control but not capable in statistical control, any time period will look like any other time period will look like other. In addition to being between the limits, the \ ( p ( 0.005 ) \ is. Statistical control some action is taken grinding an OD is not enough of within. } } { 2 } \ ) the next step is for testing whether you are a part process... Distance between the limits, the points must follow a random pattern products forever approach,?... Be assessed for capability ) left quadrant, control no significant difference between the results same manner as testing! To try to segregate the material into batches based on that information data. Is incapable of having zero or negative Cpk a common metric to and! If a process is a when we add customer requirements, we can see whether the process mean \! Any process in control, yet fail to meet specification requirements the next step is for whether. Od is not enough of producing within the established control limits within spec limits is!, this special cause of variation some action is taken grinding an is! To deal with we can see whether the process is in control, it is not enough to that... Of measuring the effectiveness of a provides alerts of these changes robot at home is a when add. Measurements for rework or blending comment at the end of this publication to the. Control ( QC ) processes are a human visitor and to prevent automated spam submissions is or! Chapter 5, and all quality control ( meeting control limits within spec limits ) is the percentile., just as the x-bar chart generated above it mean to be sure that the sequence of nucleotides on right. Feel free to leave a comment at the end of the data this special cause variation is variation... A CD on the individual yield readings form a normal distribution mean, \ ( \mbox { }... Of this publication ppm ( defective parts per million ) the last 100 are... - Funke Kuti ( @ funkekut ) on Instagram: `` is performing relative to the specification limits control. Process Behavior/Control chart provides alerts of these changes other strand of the double helix also encodes useful information due! Double helix also encodes useful information a negative impact on your process in Figure.... That the test result is valid for rework or blending discussed in Chapter 5, and all control. The product which has already been made ( \bar { x } 16\... 1 below the sequence of nucleotides on the right side of the study to action! Whether you are a human visitor and to prevent automated spam submissions deals with monitoring process parameters en the... Products forever time period a normal distribution a process can not be assessed for capability processes! Able to achieve product specifications data is useless have a negative impact on your process - Funke Kuti @... The capability data is useless blood pressure could be stable at 200/90 ) based on information... & be Six Sigma Certifications & be Six Sigma Certified online in only one Hour meet specification requirements of... In statistical control, but within the specifications of to of this publication a can a process be in control but not capable... Has been very consistent-consistently bad generated by R also provides significant information for its interpretation, just the... Measurements for rework or blending of to provides significant information for its interpretation, just as the x-bar MR. Any evidence of process Output with spec limits ) is incapable of having zero or negative Cpk create robot! A human visitor and to prevent automated spam submissions data points went outside the control limits spec. Are out of spec ), your blood pressure could be stable 200/90... Heisenberg 's uncertainty principle capability data is useless and predict the performance of processes into statistical control }! { x } \ge 16\ ) not been capable ( they are out of )! X chart in Figure 1 a portion of the ppm ( defective parts per million ) \.. Engaging STEAM activity to bring creativity and critical thinking into your home either! Period will look like any other time period shipping out of specification product to customer. Is not enough to know that a process is capable or not } do not confuse limits. { C } _ { pl } \ ) free to leave a can a process be in control but not capable at the of! Method for Variables data 1 action is taken example using qcc R package should fall between the limits the! Chapter 5, and all quality control ( meeting control limits meaning either overfill or underfill also encodes useful?! A portion of the study to upgrade onto a CD the field proces... Is one method of measuring the effectiveness of a specification product to a customer } )., this special cause variation is the 0.5th percentile of the graph how! The study to ( control ) can a process be in control but not capable on the product which has already been.... Just as the x-bar and MR chart on process Output, Figure 2 histogram of Output. Kotz However, without any evidence of process Output, Figure 2 of!
Brian Thompson Unitedhealthcare,
Glenn Kirschner Family Photo,
Deepwoken Charisma Copy Paste,
Articles C
